
PDF) Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance (MGUS)

Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukaemia - Pratt - 2009 - British Journal of Haematology - Wiley Online Library
Verification of Newly FDA-Approved Kappa and Lambda Free Light Chain Assays on a Previously Untested Platform
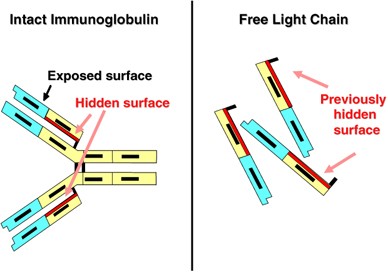
International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders | Leukemia

Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukaemia - Pratt - 2009 - British Journal of Haematology - Wiley Online Library
Serum free light chain assays (Freelite®) are recommended by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines
Monitoring serum free light chains in patients with multiple myeloma who achieved negative immunofixation after allogeneic stem cell transplantation | Haematologica
Freelite® Human Kappa Free kit For use on the Roche Cobas Integra® For in vitro diagnostic use Product Code: LK016.RI
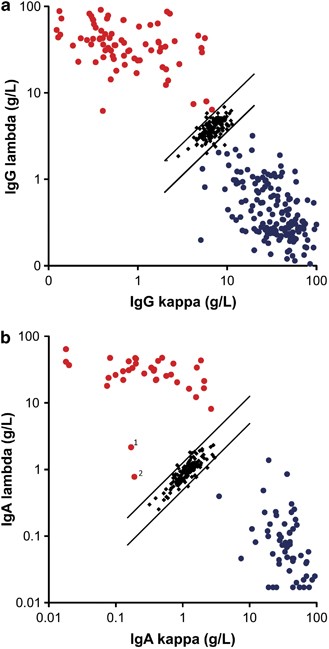
Prognostic utility of intact immunoglobulin Ig′κ/Ig′λ ratios in multiple myeloma patients | Leukemia

Comparison of two serum free light chain assays for the diagnosis of primary plasma cell malignant proliferative disease - Yang - 2019 - Health Science Reports - Wiley Online Library
Serum free light chain assays (Freelite®) are recommended by the NCCN Clinical Practice Guidelines in Oncology and the Internat
Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma | Haematologica

Comparison of Freelite™ and N Latex serum free light chain assays in subjects with end stage kidney disease on haemodialysis
Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma | Haematologica






